Sustainable Lifecycle for Plastic: Recent Advances
By Neelam Vaidya
Since the discovery of fully synthetic plastic in 1907 by Belgian chemist Leo Baekeland, many industries have found uses for this material, anywhere from its initial use as an electrical insulator to developing synthetic vascular grafts.
Today there are many types of plastics, each showing one or more essential properties like high durability, flexibility, strength, thermal, fire and chemical resistance, and dimensional stability. They are also excellent insulators and possess good optical and sound absorption qualities. As a result, plastic has become an essential part of many products from different industries.
However, due to a lack of provisions to effectively dispose or recycle it, plastic has been filling landfills worldwide. Despite the efforts to sort and recycle, less than 9% of plastic gets recycled in the U.S. with most ending up in landfill or in the environment. In 2018, landfills received 27 million tons of plastic, and 18.5% of all municipal solid waste landfill. To make matters worse, most of the plastic material is not biodegradable and contains chemicals that are known as hormone disruptors and they can leach into food and beverages.
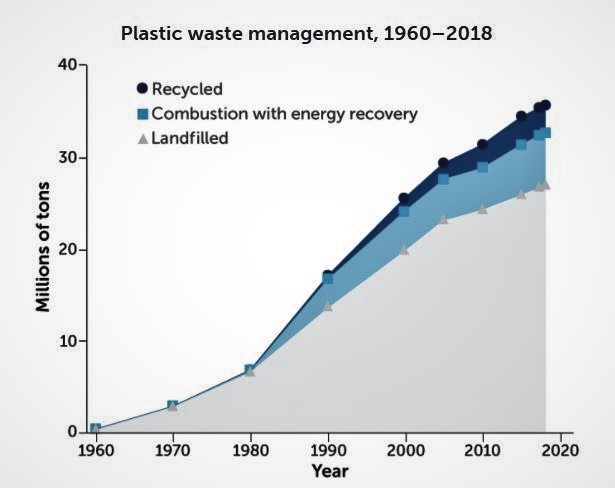
This image was obtained from the article (https://www.sciencenews.org/article/chemistry-recycling-plastic-landfills-trash-materials).
To address this out-of-control problem, scientists from different countries have been trying to find solutions that can safely recycle plastic or transform it into less harmful material. Here are three advances that researchers have made that deserve special acknowledgement and offer the potential to create more sustainable and economically favorable lifecycles for plastic.
CASE 1:
Ames Lab /Department of Energy Ames Lab discovered a process to recycle any kind of rejected plastic by modifying [a?] poly olefinic framework to generate a biodegradable surfactant/detergent-wide range of applications. They focused their work on the deconstruction of polyolefins, which represents more than half of all discarded plastics, and includes nearly every kind of product imaginable — toys, food packaging, pipe systems, water bottles, fabrics, shoes, cars, and furniture.
The process involves key steps of polymerization — the assembling of long polymer strands — but in reverse, by breaking some of the carbon-carbon bonds in the chains. The catalysts and reactions for this new process are related to those used in alkene polymerization, leveraging well-understood catalytic chemistry. Finally, the intermediates of this new transformation are easily converted into fatty alcohols or fatty acids, or used in other synthetic chemistry, to create chemicals or materials that are valuable in a whole host of ways: as detergents, emulsifiers, pharmaceuticals, and cosmetics. The best part about the process is that its end products are biodegradable.
CASE 2:
Scientists at the U.S. DOE’s Lawrence Berkeley National Laboratory (Berkeley Lab) and UC Berkeley, as reported in the journal Nature, have designed an enzyme-activated compostable plastic that could diminish microplastics pollution and holds great promise for plastics recycling. The material can be broken down to its building blocks — small individual molecules called monomers — and then reformed into a new compostable plastic product. According to Dr. Ting Xu, senior scientist in Berkeley Lab’s Materials Sciences Division, and professor of chemistry and materials science and engineering at UC Berkeley, the study was based on the fundamental principle: “Because enzymes are part of living systems, the trick would be carving out a safe place in the plastic for enzymes to lie dormant until they’re called to action.”
In a series of experiments, Xu and co-authors embedded trace amounts of the commercial enzymes Burkholderia cepacian lipase (BC-lipase) and proteinase K within the PLA and PCL plastic materials. The scientists also added an enzyme protectant called four-monomer random heteropolymer, or RHP, to help disperse the enzymes a few nanometers (billionths of a meter) apart.
In a stunning result, the scientists discovered that ordinary household tap water or standard soil composts converted the enzyme-embedded plastic material into its small-molecule building blocks called monomers and eliminated microplastics in just a few days or weeks. They also learned that BC-lipase is something of a finicky “eater.” Before a lipase can convert a polymer chain into monomers, it must first catch the end of a polymer chain. By controlling when the lipase finds the chain end, it is possible to ensure the materials don’t degrade until being triggered by hot water or compost soil.
CASE 3:
Several companies are developing methods to chemically recycle PET, including the French company Carbios, which is testing enzymes produced by microorganisms to break down PET. They used the enzyme called leaf-branch compost cutinase that is good at breaking 50% of the PET down into its monomers: ethylene glycol and terephthalic acid. To make the enzyme better at snipping apart PET, they swapped out some of the amino acids along with the PET docking site for others and found a dramatic improvement in the plastic breakdown (from 50% to 90%). Applying 3 milligrams of the enzyme per gram of PET, about 90% of the plastic broke down in about 10 hours. More importantly, by using the terephthalic acid monomers produced in that process, the researchers made new plastic bottles that were just as strong as the originals.
Reference:
- Uddhav Kanbur, Guiyan Zang, Alexander L. Paterson, Puranjan Chatterjee, Ryan A. Hackler, Massimiliano Delferro, Igor I. Slowing, Frédéric A. Perras, Pingping Sun, Aaron D. Sadow. Catalytic carbon–carbon bond cleavage and carbon-element bond formation give new life for polyolefins as biodegradable surfactants. Chem, 2021; DOI: 10.1016/j.chempr.2021.03.007
- DOE/Ames Laboratory. “Plastics could see a second life as biodegradable surfactants.” ScienceDaily. ScienceDaily, 15 April 2021. www.sciencedaily.com/releases/2021/04/210415133139.htm
- Christopher DelRe, Yufeng Jiang, Philjun Kang, Junpyo Kwon, Aaron Hall, Ivan Jayapurna, Zhiyuan Ruan, Le Ma, Kyle Zolkin, Tim Li, Corinne D. Scown, Robert O. Ritchie, Thomas P. Russell, Ting Xu. Near-complete depolymerization of polyesters with nano-dispersed enzymes. Nature, 2021; 592 (7855): 558 DOI: 10.1038/s41586-021-03408-3
- DOE/Lawrence Berkeley National Laboratory. “To design truly compostable plastic, scientists take cues from nature: New technology could steer plastics from landfills, oceans — and into your backyard compost bin.” ScienceDaily. ScienceDaily, 21 April 2021. www.sciencedaily.com/releases/2021/04/210421124624.htm
- https://www.sciencenews.org/article/chemistry-recycling-plastic-landfills-trash-materials
ViridisChem is a software company offering essential toxicity data and tools for companies to move towards sustainable product development.
We have the world’s most comprehensive toxicity database with over 90 million chemicals, and utilizing this data, our software tools provide toxicity analysis of chemicals, mixtures and formulations, and product development processes.
Demonstrations

Chemical Analyzer for Lab Safety

Chemical Analyzer for Process Development
